“Gene Action in the X-chromosome of the Mouse (Mus Musculus L.)” (1961), by Mary Lyon
In 1961, Mary Lyon, a researcher who studied genetics, published “Gene Action in the X-chromosome of the Mouse (Mus Musculus L.),” hereafter “Gene Action in the X-chromosome,” in the journal Nature. Lyon’s paper focuses on the workings of female sex chromosomes, or X-chromosomes, and their implications for gene expression. A chromosome is a structure in a cell’s nucleus that contains the DNA, or genetic information, for each individual. In her paper, Lyon proposes her X-inactivation hypothesis, which states that one of the two X-chromosomes in mammalian female cells becomes inactive during early development, silencing its genetic activity. By describing X-chromosome inactivation, Lyon provided an explanation for the mosaic patterns observed in some female mammals, where different regions of their bodies exhibit distinct traits based on the genes carried by the particular X-chromosome that is active in that region. “Gene Action in the X-chromosome” provided evidence that X-chromosome inactivation occurs, laying the basis for understanding sex-linked traits, gene expression, and X-linked genetic diseases that impact thousands of people.
Background
At the time of the article’s publication, Lyon was a researcher with the Medical Research Council, or MRC, in Harwell, United Kingdom. The MRC is an organization that funds medical research in the United Kingdom, and as of 2024, their research fundings have led to thirty-two Nobel Prize winners. Lyon earned her doctoral degree in genetics in 1950 from the University of Edinburgh in Edinburgh, Scotland. Her doctoral research focused on mice with the pallid mutation, which is when platelets, a particle that helps blood clot, do not function properly. Lyon began her postdoctoral research with the MRC in 1950 and joined its unit at Harwell in 1955. At the MRC in Harwell, Lyon began her research on mouse coat color mutations and used her findings to develop her theory of Lyonization, more commonly known as X-chromosome inactivation.
Prior to Lyon’s publication, scientists recognized there must be a biological mechanism to compensate for females having twice the number of X-chromosomes as males, but they did not know what that mechanism was. In mammals, females have two X-chromosomes, whereas males have one X-chromosome and one Y-chromosome. Females inherit one X-chromosome from their mother and the other from their father, while males inherit their X-chromosome from their mother and the Y-chromosome from their father. Each species has a different number of chromosomes. Mice, specifically, have forty chromosomes, including the sex chromosomes, or X and Y chromosomes. Each cell in an organism has its own set of chromosomes. At the time of the article’s publication, scientists were unaware as to how males, who only have one X-chromosome, have the same level of gene expression on the X-chromosome as females, who have two X-chromosomes. The exact mechanism of X-chromosome gene regulation remained unknown until Lyon published her Lyonization hypothesis in her article “Gene Action in the X-chromosome.”
Article Contents
“Gene Action in the X-chromosome” contains four untitled sections. Lyon begins with an introduction, in which she outlines that the two X-chromosomes in females have distinct structures. Then, in section two, she describes her Lyonization Hypothesis, where she writes that one X-chromosome in every cell in female mice is always inactivated. Next, in section three, Lyon explains that prior to proposing her hypothesis, she studied mosaic coat patterns in mice and found that the patterns occur due to a mix of the inactivation of some normal X-chromosomes and some mutant X-chromosomes in early development, which provided evidence that X-inactivation occurs. In the conclusion of her article, she hypothesizes that X-inactivation might occur in cells during the blastocyst stage, which is an early stage in development in which an embryo consists of a ball of cells. She also claims that sex chromatin, a small, condensed structure in female cells, is the inactive X-chromosome in all female mammals, not just in rats and opossums as previously observed by scientists.
In the introduction of her article, Lyon begins by briefly describing the work of researchers Susumu Ohno and Theodore S. Hauschka in their 1960 article, “Allocycly of the X-chromosome in Tumors and Normal Tissues.” Lyon explains that Ohno and Hauschka found that in female mice, the first X-chromosome in cancer, normal ovary, mammary gland, and liver cells appeared different from the second X-chromosome in each cell. That one chromosome was heteropyknotic, meaning that the chromosome appeared darker or more tightly packed and coiled under a microscope than a regular chromosome. According to Lyon, they realized that what scientists knew as the sex chromatin structure was actually a condensed X-chromosome in mice. Although Ohno and Hauschka identified that the two X-chromosomes in females exhibit different coiling behaviors, Lyon writes that Ohno and Hauschka’s article left unresolved whether the heteropyknotic chromosome was the paternally or maternally inherited X-chromosome (Figure 1).
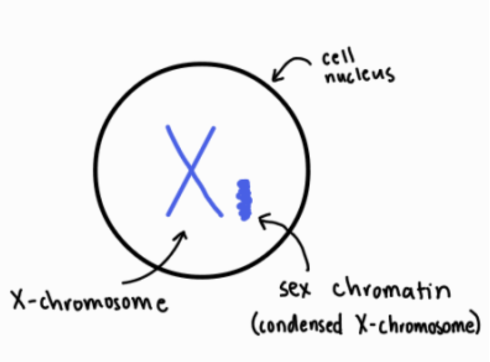
In the second section, Lyon expands upon the available evidence from Ohno and Hauschka to develop her Lyonization hypothesis, which consists of two facets. First, Lyon argues that the heteropyknotic X-chromosome can come from either the mother or the father in different cells of the same organism. Second, Lyon writes that the heteropyknotic X-chromosome is inactive, which means that it does not have any genetic activity. Those two ideas made up her inactivation hypothesis, which, as of 2025, is known as the Lyonization or X-inactivation hypothesis.
In the third section, Lyon provides evidence to support her hypothesis that there is one inactivated X-chromosome in mammalian female cells, and the first piece of evidence is that female mice born with only one X-chromosome, labeled as XO females, develop normally. According to Lyon, that evidence demonstrates that mammalian females only need one working, or active, X-chromosome in their cells to reproduce and exhibit normal development.
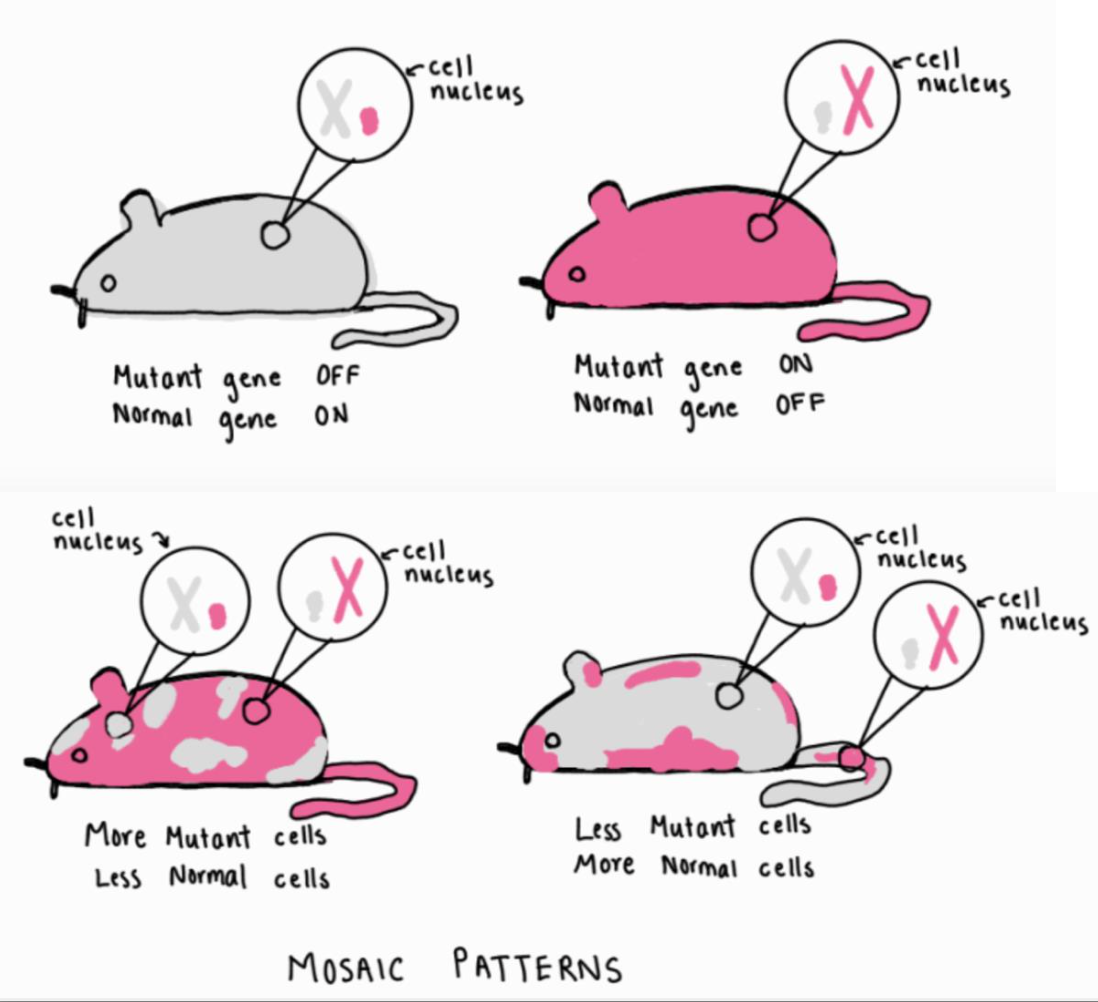
Continuing in the same section, Lyon explains that the second piece of evidence supporting her hypothesis concerns coat color mutations in female mice. She details that all female mice with a sex-linked coat color mutation have patches of different colors on their coats known as a mosaic pattern. A sex-linked mutation occurs when a gene on the X or Y chromosome is different from the typical gene sequence. Sometimes, coat color genes on each of the two X-chromosomes in female mice are different because one X-chromosome will have a typical version of the gene that codes for one coat color, and the other X-chromosome will have a mutated version of the gene that codes for a different color. Lyon argues that the mosaic coat color pattern in female mice then occurs due to the inactivation of one of their X-chromosomes during early embryonic development. If the X-chromosome with the mutant gene turns off, leaving only the typical X-chromosome activated, that area on the mouse will have a typically colored patch. If the X-chromosome with the typical gene turns off, leaving only the mutated X-chromosome activated, that area of fur will be a different, or mutant, colored patch. Lyon also writes that sometimes there may be patches that resemble an intermediate color between the typical and mutated coat colors because different cells can mix together when they are growing, causing both colors to be present in the same area. She explains that the sex-linked gene for hair structure, known as tabby, in female mice also works in a similar manner, providing further evidence in support of her Lyonization hypothesis.
Next, in the third section, Lyon explains that her X-inactivation hypothesis demonstrates that all sex-linked genes in which the physically expressed trait is due to localized gene action result in a mosaic pattern for mice that have one copy of the typical gene and one copy of the mutant gene on different X-chromosomes. Localized gene action refers to the phenomenon where specific genes are only expressed in particular regions of an organism, and thus, can only affect the physical appearance of those specific regions. For example, if a mouse has a mosaic coat pattern, they exhibit localized gene action because different regions of their fur can express different physical colors. She also states that the mosaic pattern that organisms exhibit depends on the ratio between the cells with the active mutated gene and cells with the active typical gene. For instance, if there are more typical cells present, the mouse may look more typical, and if there are more mutant cells present, the mouse may look more mutant, but never entirely one or the other. Because the mosaic patterns vary per mouse and depends on the ratio of cells, it can be difficult to predict the mouse’s overall physical appearance (Figure 2).
Lyon ends section three by providing more examples of mice genes, including the bent-tail and Jimpy gene, that reflect how X-inactivation can impact the physical expression of genes. Certain variations of a gene, known as alleles, can be dominant or recessive. When an allele is dominant, the characteristics produced by that allele will always appear if that allele is present. When an allele is recessive, the characteristics of that allele will only show if the dominant version of the gene is completely absent. Lyon provides an example where she describes that about ninety-five percent of mice with one typical and one mutant copy of a gene called bent-tail will look like they have the mutant gene. The mutant copy of the bent-tail gene is the dominant allele and will almost always show up physically if present. In contrast, even if a majority of a mouse’s cells contain an activated mutant gene called Jimpy, as long as the typical gene is active in at least a few cells, the mutant gene will become inactive, causing the mice to have a typical physical appearance. In that case, the typical allele of the gene is dominant and will mask the mutant gene whenever the dominant allele is present. However, Lyon cites another researcher, R. J. Phillips, who found a rare case where a female mouse expressed the Jimpy gene despite only having one copy of the typical dominant gene that should have been the expressed feature. She argues that the phenomena could be because, by chance, all the cells causing the Jimpy effect had their typical gene turned off, so the mouse only expressed the mutant Jimpy gene.
In the fourth section, Lyon proposes that X-chromosome inactivation occurs during the blastocyst stage in mice. She initially explains that all the gathered evidence during the time of publication does not provide a concrete answer to exactly when X-chromosome inactivation occurs in mice. Lyon mentions that in cat, monkey, and human embryos, sex chromatin forms during the late blastocyst stage, which occurs approximately five to six days after fertilization as cells continue to divide and replicate to create the embryo. She suggests that X-chromosome inactivation occurs at a similar stage, according to her research. A female mouse with only one working X-chromosome is typically fertile, so Lyon proposes that both X-chromosomes do not need to be active when the reproductive organs are forming, indicating that X-chromosome inactivation can indeed occur early on in embryonic development. As of 2025, researchers agree with Lyon’s hypothesis that X-inactivation occurs during the blastocyst stage.
Lyon finishes her article by claiming that the sex chromatin structure is the condensed X-chromosome in all mammals. She states that scientists already believed that sex chromatin is an X-chromosome in rats and opossums, but scientists did not know whether sex chromatin was an X-chromosome in all female mammals. Lyon states that if sex chromatin is an X-chromosome across all mammals, then all female mammals with a mutant gene and typical gene for a sex-linked gene would show the same patterns as what she observed in female mice. To support her theory and conclude the article, she refers to the coat of the tortoiseshell cat and its mosaic black and yellow pattern, which fulfills the expectations of her theory.
Impacts
Following the article’s publication, most scientists accepted Lyon’s work and her X-chromosome inactivation hypothesis, but others, such as Hans Grüneberg, a researcher studying animal genetics, criticized her work. Grüneberg published multiple articles describing his criticisms of the Lyonization hypothesis. For example, in 1966, he published an article describing how the sex-linked gene for tabby, which correlates with a dental syndrome in mice, does not support Lyon’s hypothesis. He wrote that some mice with one copy of the typical gene and one copy of the mutant gene did not always display a mosaic pattern. Instead of only displaying a mix of both traits, some mice had all normal teeth, while others only had teeth with the dental syndrome associated with the tabby gene. According to Manuel Ruiz Rejón, a professor of genetics at the University of Granada in Granada, Spain, the scientific community widely accepted X-chromosome inactivation despite Grüneberg and other researchers’ criticism.
As of 2025, scientists continue to accept the Lyonization hypothesis that Lyon outlined in “Gene Action in the X-chromosome,” and her hypothesis has helped researchers to understand the inheritance of X-linked diseases. Common X-linked diseases include Duchenne muscular dystrophy, which causes progressive muscular weakness, and hemophilia, a genetic bleeding disorder affecting over 1.1 million people worldwide that causes a person’s blood to not clot properly. Because males only have one X-chromosome, if the mutation for an X-linked disease is present on their X-chromosome, the male will inherit the disease. However, since females have two X-chromosomes and one is inactivated, the female may not have the disease, even if one of their X-chromosomes contains the mutation. A female who does not have symptoms but does have the disease mutation on one of their X chromosomes, is known as a carrier. Understanding female carriers through X-chromosome inactivation helps scientists to research the mechanisms behind X-linked diseases, create diagnostic tests, and develop potential treatments.
Although Lyon explained that X-inactivation occurs, she did not provide a mechanism to explain what initiates it. Around thirty years after Lyon published her Lyonization hypothesis, researchers also used her hypothesis to aid in the discovery of X-inactive specific transcript, or the Xist gene, a non-coding RNA, or ncRNA, that initiates and regulates X-chromosome inactivation. The discovery of that gene and others eventually revealed that a majority of the human genome consists of DNA sequences that produce ncRNAs rather than proteins.
As of 2025, over 4,900 publications have cited “Gene Action in the X-chromosome,” according to Google Scholar. Many of those articles argue that DNA methylation, a process that can change the activity of a DNA segment without altering its sequence, maintains X-inactivation. DNA methylation is similar to a switch that can turn genes on or off, and it controls what type of cell each individual cell differentiates into. For example, one article that cites “Gene Action in the X-chromosome” found that activated X-chromosomes genes have lower levels of DNA methylation at the beginning of the DNA sequence, while inactivated X-chromosomes genes have increased levels of DNA methylation at the same location. Therefore, DNA methylation and X-chromosome inactivation are correlated.
“Gene Action in the X-Chromosome” was one of the first articles to propose the X-inactivation mechanism, a concept which researchers continue to accept, as of 2025. Lyon’s theory and research as explained in the article continues to shape the way scientists comprehend sex-linked genetic traits and gene regulation.
Sources
- Cleveland Clinic. “Blastocyst.” Cleveland Clinic. Last modified April 29, 2022. https://my.clevelandclinic.org/health/body/22889-blastocyst (Accessed July 18, 2024).
- Grüneberg, Hans. “The Molars of the Tabby Mouse, and a Test of the ‘Single-active X-chromosome Hypothesis.” Journal of Embryology and Experimental Morphology 15 (1966): 223–4.
- Haines, Catharine M. “Lyon, Mary Frances.” Oxford Dictionary of National Biography. https://www.oxforddnb.com/display/10.1093/odnb/9780198614128.001.0001/odnb-9780198614128-e-109090 (Accessed July 16, 2024).
- Hemophilia Federation of America. “Hemophilia A.” Hemophilia Federation of America. https://www.hemophiliafed.org/disease_type/hemophilia-a/ (Accessed July 18, 2024).
- Loda, Agnese, and Edith Heard. “Xist RNA in Action: Past, Present, and Future.” PLoS Genetics 15 (2019): e1008333. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6752956/ (Accessed July 18, 2024).
- Lyon, Mary Frances. “Gene Action in the X-Chromosome of the Mouse (Mus Musculus L.).” Nature 190 (1961): 372–3. https://www.nature.com/articles/190372a0.pdf (Accessed July 18, 2024).
- Ohno, Susumu and Theodore S. Hauschka. “Allocycly of the X-chromosome in Tumors and Normal Tissues.” Cancer Research 20 (1960): 541–5. https://aacrjournals.org/cancerres/article/20/4/541/474395/Allocycly-of-the-X-Chromosome-in-Tumors-and-Normal (Accessed July 16, 2024).
- Rastan, Sohaila. “Mary F. Lyon (1925-2014).” Nature 36 (2015).
- Rejón, Manuel Ruiz. "Mary Lyon: The Geneticist Who Discovered That Women Are (Cellular) Mosaics." OpenMind, July 2, 2020. https://www.bbvaopenmind.com/en/science/leading-figures/mary-lyon-geneticist-discovered-women-are-cellular-mosaics/ (Accessed July 16, 2024).
- Sharp, Andrew J., Elisavet Stathaki, Eugenia Migliavacca, Manisha Brahmachary, Stephen Montgomery, Yann Dupre, and Stylianos E. Antonarakis. “DNA Methylation Profiles of Human Active and Inactive X Chromosome.” Genome Research 21 (2011): 1592–https://genome.cshlp.org/content/21/10/1592.short (Accessed July 18, 2024).
- UK Research and Innovation. “Who MRC Is.” UK Research and Innovation. https://www.ukri.org/who-we-are/mrc/who-we-are/ (Accessed July 16, 2024).
- University of Manchester. “Medical Research Council (MRC): Evaluation of Early Careers Programme.” University of Manchester. https://documents.manchester.ac.uk/display.aspx?DocID=59030 (Accessed July 16, 2024).
Keywords
Editor
How to cite
Publisher
Handle
Rights
Articles Rights and Graphics
Copyright Arizona Board of Regents Licensed as Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported (CC BY-NC-SA 3.0)